ÉQUIPE 5 – Fabienne Foufelle – Maladies métaboliques, diabète et comorbidités

The team “Metabolic diseases, diabetes and co-morbidities” (MEDICIS) has three objectives: (i) the study of type 2 diabetes comorbidities, such as insulin resistance and Non Alcoholic Fatty Liver Disease and their relationship with lipid metabolism (ii) the role of the mineralocorticoid receptor in cardiometabolic and cardiorenal diseases and (iii) the mechanisms of cardiac remodeling during aging. The group led by F. Foufelle studies the complex relationship between “Metabolic Dysfunction Associated Steatotic Liver Disease” (MASLD) and type 2 diabetes and the mechanisms of lipid-induced insulin resistance, a key feature of type 2 diabetes. It is particularly interested (i) by the multi-faceted role of mitochondria in the progression of MASLD and hepatic fibrosis (ii) by the role of sphingolipid species (ceramides, dihydroceramides and sphingosine 1P), in hepatic metabolism and insulin resistance (and (iii) by the role of endoplasmic reticulum stress in Metabolic dysfunction-Associated Steatohepatitis (MASH). There is a strong continuum between basic research and clinic, thanks to the presence of both researchers and clinicians (diabetology and hepatology departments of La Pitié Salpêtrière hospital). The group led by F. Jaisser focuses on the understanding of the signaling and pathophysiological roles of the hormone aldosterone and its receptor, the mineralocorticoid receptor (MR), with a mainstream objective of translating preclinical data to clinical studies including the development of interventional clinical trials when possible. The group developed several rodent and pig models to assess the roles of MR and its downstream targets in the renal and cardiovascular fields and beyond (i.e liver, retina, skin and adipose tissues for example). Novel MR downstream targets such as Neutrophil Gelatinase associated Lipocalin were identified and their role is presently studied, particularly in MASLD. The repositioning of MR antagonists is studied in seminal translational research in collaboration with several pharma companies. S. Besse is investigating the mechanisms responsible for the transition to heart failure in old age and particularly the NF-κB and FoxO signaling pathways in the rodent and human heart.
 Four main axes are developed:
Four main axes are developed:
1- Sphingolipids and insulin resistance
2- Inter-organ dialogue in metabolic pathologies.
3- Cellular and molecular mechanisms of steatosis and steatohepatitis
4- Heart, kidney and mineralocorticoids
THEME 1. Sphingolipids and insulin resistance

Eric HAJDUCH
Insulin resistance is a pathological condition in which insulin sensitive organs, liver, muscles, adipose tissue do not respond well to the action of insulin, an hormone produced by pancreatic ß cells. Normally, insulin facilitates the entry of glucose into muscle and adipose cells, inhibits the production of glucose by the liver and promotes lipid storage in adipose tissue while slowing their breakdown (lipolysis). In case of insulin resistance, cells no longer respond effectively to insulin, blood glucose levels remain high (hyperglycemia), and the pancreas compensates with an hyperinsulinemia (overproduction of insulin), eventually exhausting the pancreatic ß- cells. Possible metabolic consequences include chronic hyperglycemia, hyperinsulinemia, hepatic steatosis, dyslipidemia, hypertension, and the potential development of type 2 diabetes (T2D).
Factors contributing to insulin resistance include overweight and abdominal obesity, a sedentary lifestyle, a diet high in simple sugars and saturated fats, chronic inflammation, and gut microbiota imbalance. In the context of obesity, a main risk factor for T2D, non-adipose tissues such as skeletal muscle and the liver accumulate lipids among which sphingolipid derivatives such as ceramides. Ceramides are among the most active lipid messengers for the inhibition of key proteins in the insulin signaling pathway, thus inducing insulin resistance in these tissues.
Our objectives are to understand the mechanisms by which sphingolipids produced by steatotic livers impact on peripheral metabolism. We are studying ceramides associated with lipoproteins found in the bloodstream, whose concentrations are known to increase significantly under conditions of MASLD.
Some studies have shown that LDL-associated ceramides (Low Density Lipoproteins)can exert deleterious effects on muscle metabolism. We want to characterize the ceramide species involved (e.g. short or long chain) and the mechanisms involved (role of the LDL receptor, cellular ceramide metabolism…) in in vivo and in vitro studies (cultured myotubes). While the role of ceramides in the development of insulin resistance and cardiovascular disease is now well established, the role of another sphingolipid called sphingosine-1- phosphate (S1P) remains elusive. S1P is produced by the liver and by platelets and is associated with HDL lipoproteins (High density Lipoproteins) or with albumin. Obesity and diabetes are associated with increased concentrations of ceramide-containing LDL, but also with decreased concentrations of HDL-S1P. HDL-S1P has been shown to promote the function and survival of pancreatic β cells and may also enhance insulin signaling. Obesity-induced insulin resistance reduces HDL-S1P levels. Our project aims at understanding the involvement of this sphingolipid in metabolic diseases (MASLD, obesity, diabetes) and the underlying mechanisms in mice, as well as in humans.
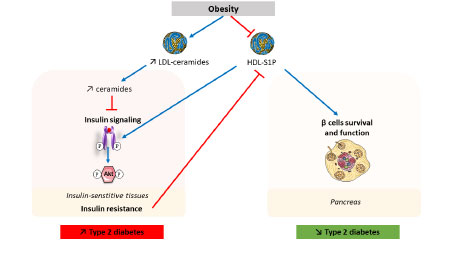
Involvement of sphingolipids in type 2 diabetes
THEME 2. Inter-organ dialogue in metabolic pathologies

Franck Phan et Olivier Bourron
Chronic metabolic diseases, such as obesity and type 2 diabetes (T2D), are rapidly increasing worldwide. They are frequently associated with MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease), reflecting the liver involvement in metabolic syndrome. These three conditions are closely intertwined: obesity and T2D are major risk factors for the development of MASLD, while the progression of MASLD can, in turn, worsen overall metabolic homeostasis. When not properly managed, T2D can lead to chronic complications, particularly cardiovascular and peripheral neurological, which severely impact patients’ quality of life and worsen their prognosis. Far from resulting from isolated metabolic dysfunctions, these diseases are based on a systemic and integrated pathophysiology that extends beyond the “classic metabolic organs” such as the liver, adipose tissue, or muscle. Indeed, these disorders are accompanied by a complex imbalance in inter-organ exchanges, mediated by a wide variety of bioactive signals—lipids, cytokines, metabolites, and hormonal peptides—which orchestrate a pathological inter-tissue dialogue.
Understanding the molecular and cellular mechanisms that govern these inter-organ interactions is a major challenge for identifying new therapeutic targets and designing more integrated approaches to the prevention and treatment of these metabolicdiseases and their complications. Our research program is aligned with this systemic approach, exploring the functional interactions between the liver, adipose tissue, heart, and the arterial and peripheral nervous systems in the context of metabolic disturbances such as MASLD, type 2 diabetes, and obesity. The originality of our approach lies in its strong integration of basic research, experimental modeling, and clinical investigation, with a clear commitment to rapid translation into clinical practice. Thanks to the use of cutting-edge techniques—such as lipidomics, metabolomics, transcriptomics, and functional physiological studies—and the availability of human cohorts, our work aims to decipher how signals emitted by an altered organ (such as a steatotic liver or inflammatory adipose tissue) can trigger pathological responses in other tissues, including the heart, arteries, and peripheral nervous system, thus contributing to the development and worsening of complications associated with metabolic syndrome and diabetes. Among our key projects: The NADRANK project aims to better understand the molecular exchanges between adipose tissue and the liver and their impact on the development and progression of MASLD and its systemic complications. The CONNECT project explores the link between liver damage and cardiac dysfunction in MASLD, through the study of circulating signaling molecules of hepatic origin that can modulate inflammation, fibrosis, or myocardial remodeling. The SYNAPSE project investigates the molecular mechanisms underlying peripheral neuropathies in patients with MASLD and/or type 2 diabetes, aiming to better understand this frequent complication for which there is currently no curative treatment. All of these projects benefit from a strong hospital-university foundation, working closely with the clinical departments of the Pitié-Salpêtrière Hospital – APHP (Diabetology, Hepatology, Cardiology) and the technical platforms of Sorbonne University and INSERM. Our ability to connect molecular, tissue, and clinical levels within a systemic view of metabolic diseases constitutes a significant added value for the successful completion of our research program.
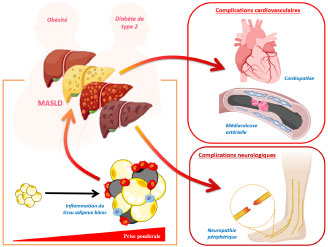
Inter-organ interactions and systemic complications in metabolic diseases: focus on adipose tissue, the liver, the heart, and the peripheral nervous system
THEME 3. Cellular and molecular mechanisms of steatosis and steatohepatitis

Fabienne Foufelle Marie Lagouge
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a liver disease whose incidence is steadily increasing worldwide. MASLD is a comorbidity strongly associated with type 2 diabetes and obesity. Its prevalence is estimated at 30% in the general population and over 80% in obese individuals. MASLD encompasses a spectrum of pathologies ranging from simple steatosis, characterized by lipid accumulation in hepatocytes, to steatohepatitis (MASH), whose main characteristics are inflammation and fibrosis. Fibrosis can progress to more severe pathologies such as cirrhosis and hepatocellular carcinoma (HCC), and is also strongly linked to cardiovascular disease. Our team has an extensive expertise in studying liver diseases associated with type 2 diabetes, and particularly aspects of steatosis involving lipid metabolism.
In recent years, our projects have focused on studying the progression of steatosis to more severe stages of MASLD, such as MASH. We are particularly interested in the cellular and molecular mechanisms that control the activity of different liver cell types, namely hepatocytes, the predominant cells and key metabolic players in the liver, as well as hepatic stellate cells, which are responsible for fibrogenesis. Our research focuses particularly on the lipid metabolism of these cells in relation to the activity of the endoplasmic reticulum and mitochondria. Our current key projects are: The IRE1 NASH project (to be completed) the IMHOTEP project, which addresses the role of hepatocyte mitochondrial dysfunction in the onset and progression of MASLD, the MITOSTAR and CEMPR projects, which respectively address the role of mitochondrial activity in the transdifferentiation of hepatic stellate cells and hepatic fibrogenesis and the importance of endoplasmic reticulum-mitochondria contact in hepatic stellate cells during the progression and regression of MASLD.” All of these studies combine physiological studies of preclinical models and cellular and molecular explorations, allowing us to deepen our knowledge of the role played by intracellular organelles (endoplasmic reticulum and mitochondria) in the onset and progression of MASLD.
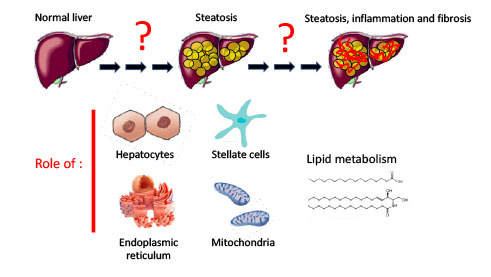
What are the actors and mechanism of MASLD/MASH ?
THEME 4. Heart, kidney and mineralocorticoids

Fréderic JAISSER et Sophie BESSE
Our goal is to improve our understanding of the pathophysiological role of signaling pathways through which cardiac aging and the mineralocorticoid receptor (MR) promote cardiac pathologies in the context of metabolic and renal diseases. Our work combines cellular and molecular approaches, animal physiology and pharmacological studies. It also includes translational research aimed at identifying and validating in humans the signaling pathways demonstrated in cellular or animal models.
Our objective is also to strengthen the rationale for developing new therapeutic applications of mineralocorticoid receptor antagonists. We aim at understanding the mechanisms responsible for cardiac remodeling during aging. In a human atrial tissue model, we are exploring the relative contribution of different cell death pathways and their control mechanisms, leading to the depletion of the pool of viable cardiomyocytes during aging and to the remodeling of the extracellular matrix. A second area of research concerns the involvement of the MR in cardiorenal syndrome, particularly heart failure with preserved ejection fraction and aortic valve disease, both of which are associated with chronic kidneydisease. We have identified neutrophil gelatinase-associated lipocalin (NGAL) as a downstream target of the MR, whose role is critical in the deleterious effects of MR activation in these pathologies. A third area of research, conducted in collaboration, explores the role of the MR in various extrarenal or extracardiac pathologies, for example, in ophthalmology, chronic inflammatory bowel diseases, or the oral cavity. Finally, several partnerships have been established with major pharmaceutical companies to characterize new non-steroidal MRs and their benefits in various comorbidities.
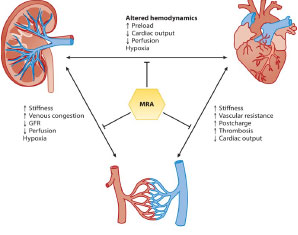
Beneficial effects of mineralocorticoid receptor (MRA) antagonists in nephro-cardiology

| Name | Position | ORCID |
|---|
- Le Goff W, BOURRON O, Materne C, Galier S, PHAN F, Tan-Chen S, Guillas I, HARTEMANN A, Salem JE, Redheuil A, FOUFELLE F, Le Stunff H, HAJDUCH E, Guerin M. Inverse relationship between circulating sphingosine-1-phosphate and precursor species and coronary artery calcification score in type 2 diabetes. Cardiovasc Diabetol. 2025 Feb 21;24(1):85.
- DENIMAL D, Ponnaiah M, PHAN F, Jeannin AC, Redheuil A, Salem JE, Boussouar S, Paulstephenraj P, Laroche S, Amouyal C, HARTEMANN A, FOUFELLE F, BOURRON O. Metabolic dysfunction-associated steatotic liver disease (MASLD) biomarkers and progression of lower limb arterial calcification in patients with type 2 diabetes: a prospective cohort study. Cardiovasc Diabetol. 2025 Apr 23;24(1):176.
- JAISSER F, BARRERA-CHIMAL J. Mineralocorticoid receptor antagonism for non-diabetic kidney disease. Nephrol Dial Transplant. 2025 Feb 5;40:i29-i36.
- Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, Anstee QM, Abdelmalek MF, Younossi Z, Baum SJ, Francque S, Charlton MR, Newsome PN, Lanthier N, Schiefke I, Mangia A, Pericàs JM, Patil R, Sanyal AJ, Noureddin M, Bansal MB, Alkhouri N, Castera L, Rudraraju M, RATZIU V; MAESTRO-NASH Investigators. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N Engl J Med. 2024 Feb 8;390(6):497-509.
- BONNARD B, El Moghrabi S, Ueda K, Lattenist L, Soulie M, López-Andrés N, Xhaard C, Shimosawa T, Rossignol P, JAISSER F. NGAL is a Novel Target in Hypertension by Modulating the NCC-Mediated Renal Na Balance. Hypertension. 2023 Sep;80(9):1860-1870.
- Bandet CL, Tan-Chen S, Ali-Berrada S, Campana M, Poirier M, Blachnio-Zabielska A, Pais-de-Barros JP, Rouch C, Ferré P, Foufelle F, Le Stunff H, Hajduch E. Ceramide analog C2-cer induces a loss in insulin sensitivity in muscle cells through the salvage/recycling pathway. J Biol Chem. 2023 Jun;299(6):104815.
- HAJDUCH E, LACHKAR F, FERRE P, FOUFELLE F. Roles of Ceramides in Non-Alcoholic Fatty Liver Disease. J Clin Med. 2021 Feb 16;10(4):792.
- CARLIER A, PHAN F, SZPIGEL A, HAJDUCH E, Salem JE, Gautheron J, Le Goff W, Guérin M, LACHKAR F, RATZIU V, HARTEMANN A, FERRE P, FOUFELLE F, BOURRON O. Dihydroceramides in Triglyceride-Enriched VLDL Are Associated with Nonalcoholic Fatty Liver Disease Severity in Type 2 Diabetes. Cell Rep Med. 2020 Dec 22;1(9):100154.
- BOURRON O, PHAN F, Diallo MH, Hajage D, Aubert CE, CARLIER A, Salem JE, Funck-Brentano C, Kemel S, Cluzel P, Redheuil A, DAVAINE JM, Massy Z, Mentaverri R, Bonnefont-Rousselot D, Gillery P, Jaisson S, Vermeer C, Lacorte JM, Bouziri N, Laroche S, Amouyal C, HARTEMAN A. Circulating Receptor Activator of Nuclear Factor kB Ligand and triglycerides are associated with progression of lower limb arterial calcification in type 2 diabetes: a prospective, observational cohort study. Cardiovasc Diabetol 2020 Sep 18;19(1):140.
- BANDET CL, MAHFOUZ R, Véret J, Sotiropoulos A, POIRIER M, Giussani P, Campana M, Philippe E, Blachnio-Zabielska A, BALLAIRE R, LE LIEPVRE X, BOURRON O, Berkeš D, Górski J, FERRE P, Le Stunff H, FOUFELLE F, HAJDUCH E. Ceramide Transporter CERT Is Involved in Muscle Insulin Signaling Defects Under Lipotoxic Conditions. Diabetes. 2018 Jul;67(7):1258-1271.
- Besse S, Nadaud S, Balse E, Pavoine C. Early Protective Role of Inflammation in Cardiac Remodeling and Heart Failure: Focus on TNFα and Resident Macrophages.
Cells. 2022, 11(7):1249.
 |
Université Paris Saclay, Université Paris Cité, Université Côte d’Azur, Université de Dijon, Université de Lorraine, CIC CHU Nancy Brabois, Université de Compiègne, Université de Rouen. |
 |
Bialystok University, Poland; Universidad de Valladolid, Spain; Universidad Pública de Navarra, Pamplona, Spain; DZHK (German Centres for Cardiovascular Research). |
 |
Melbourne University, Australia; Augusta University, USA; Université de Montréal, Canada; San Sebastián University, Chile. |
 |
Bayer SAS, AstraZeneca, Poxel, Enterome. |
The team is a member of the IHU “ICAN” and the Fédération Hospitalo-Universitaire – Groupe Transplantation Ile De France
- Frédéric JAISSER Inhibitors of NGAL protein WO 2020/1784412019 INSERM, Université de Rouen Normandie, Greenpharma S.A.S, Sorbonne Université, UNIVERSITE DE PARIS
- Olivier BOURRON, Agnès HARTEMANN Method for diagnosis and treating peripheral neuropathies Brevet Europe EP 18306503.6 2018 Sorbonne Université Pr. Bourron is the laureate of the 2020 innov APHP trophy



