TEAM 4 – Wilfried Le Goff et Maryse Guerin- Cellular and Systemic Lipid Metabolism in Cardiometabolic Diseases

Wilfried Le Goff, Team 4 Head
OBJECTIVES
Cardiovascular diseases still remain the major cause of morbidity and mortality worldwide due to the growing prevalence of obesity and associated metabolic disorders, including insulin resistance and Type 2 diabetes. Dyslipidemia, characterized by altered circulating concentrations of lipoproteins and lipids is a major component in the development of cardiometabolic diseases (CMD). As a consequence, lipid-lowering therapies are the privileged therapeutic strategy in CMD. Mechanisms through which lipids contribute to the development of metabolic disorders are multiple and involve complex signaling and regulation pathways at the both cellular and systemic levels. Importantly, it is now clear that a large spectrum of lipid species not only restricted to cholesterol and triglycerides participates actively in alterations of lipid and lipoprotein metabolism in CMD. Then, deciphering of dysfunctional lipid metabolic pathways might help to propose new therapeutic targets to prevent or hamper the occurrence and development of CMD.
Our recent pioneering studies leading to the identification by omics approaches of lipid networks and metabolic pathways controlling biological activities of plasma lipoproteins and cell activation in CMD open up new insights in understanding how alterations in lipid metabolism contribute to CMD onset. In particular, our recent findings lead us to revisit the current understanding of atheroprotective high-density lipoprotein (HDL) and triglyceride-rich lipoprotein (TRL) metabolism and its role in CVD.
Building on tight interactions with clinical and valorization departments, our research program aims to propose new candidate pathways, genes and biomarkers in the context of CMD.
THEME 1. Deciphering molecular mechanisms controlling cell and tissue lipid homeostasis in cardiometabolic diseases – Wilfried Le Goff
Maintenance of cellular lipid homeostasis is essential for preservation of tissue function especially in metabolic states characterized by a disturbance of lipid metabolism. Thus, alteration of lipid homeostasis in key metabolic tissue such as liver, adipose tissue and intestine, but also in vascular wall, is a frequent feature of cardiometabolic diseases (CMD). Macrophages are pivotal actors in the maintenance of tissue lipid homeostasis and activation of macrophages by bioactive lipids is a critical event in development and progression of these pathologies. Our previous works aiming the study of mechanisms governing macrophage lipid homeostasis uncovered a tight link between the membrane ATP-Binding Cassette G1 (ABCG1) lipid transporter and cellular triglyceride storage in CMD. Whereas we demonstrated that ABCG1 is not a significant actor in cholesterol efflux from human macrophages (Larrede S., 2009), this function being mostly carried by ABCA1 (Du XM, 2015), we identified a new role for ABCG1 in foam cell formation through the control of the bioavailability of lipoprotein lipase in macrophages (Olivier M., 2012). We extended our work in the context of obesity and revealed the contribution of adipose ABCG1 in triglyceride storage, fat mass growth and obesity in humans (Frisdal E., 2015). Importantly, the manipulation of ABCG1 brought to light the intimate link between sphingomyelin and triglycerides storage. We propose that ABCG1 could be a potential therapeutic target in obesity (PCT/EP2011/073140) and we are currently evaluating the impact of the ABCG1 targeting on diet-induced obesity and associated metabolic disorders.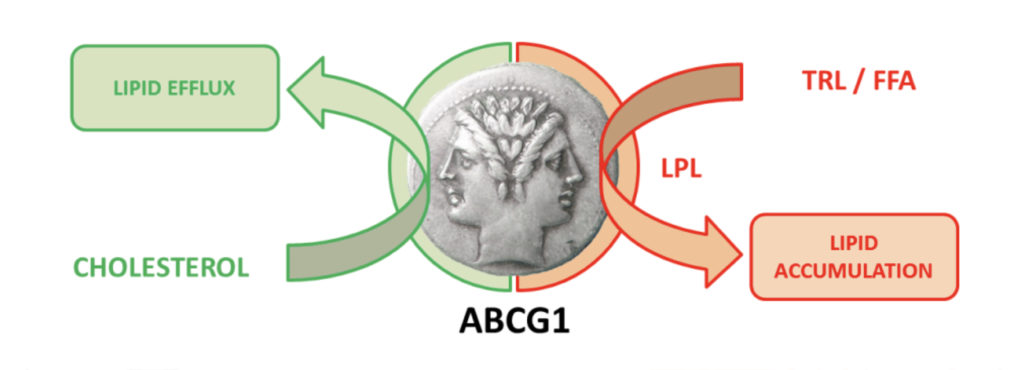
ABCG1 is a Janus-faced metabolic switch
In a cholesterol-rich metabolic context, ABCG1 protects from tissue lipid accumulation by promoting cellular free cholesterol efflux (left side of the Janus face).
By contrast, ABCG1 promotes cellular lipid storage in a fat-rich environment by controlling lipoprotein lipase activity (right side of the Janus face).
TRL: Triglyceride-rich lipoproteins, FFA: Free fatty acids, LPL: lipoprotein lipase.
From Frisdal E. & Le Goff W. Adipocyte, 2015.
Beyond sphingomyelin, sphingolipids and phospholipids have emerged as being intimately associated to the appearance of cardiometabolic disorders. However, the mechanisms through which those lipids exert their action in the context of CMD are complex and largely unknown. More specifically, sphingolipids and phospholipids are essential in the architecture of the membrane structure which is a critical pillar in macrophage functions. Dietary fatty acids can be metabolized into a range of sphingolipids and phospholipids species; however the impact as well as the precise mechanisms involved in such a rearrangement in macrophages are not yet elucidated. Building on the pioneering characterization of the lipidome and transcriptome of tissue macrophages in response to dietary lipids, our group aims to determine how the tissue and metabolic shaping of macrophages by sphingolipids and phospholipids contributes to the appearance of CMD disorders. This project will help to decipher the complex interactions between diet, lipid metabolism and metabolic diseases by delineating the cellular regulatory pathways by which sphingolipids and phospholipids exert their biological activities in the context of CMD. In addition, this project will allow the identification of potential new therapeutic targets as well as new circulating biomarkers in the treatment and the prevention of CMD.
THEME 2. Exploration of the close link between HDL phenotype and lipid-laden macrophage / histiocyte formation for the identification of new physiopathological pathways in rare human diseases – Wilfried Le Goff
Alteration of cellular lipid homeostasis is frequently observed in numerous pathologies although this latter do not result primarily from major defects of lipid metabolism but rather is a secondary clinical manifestation of the disease. Such a disturbance is associated with autoimmune or inflammatory diseases such as rheumatoid arthritis (RA), Systemic Lupus Erythematosus (SLE), Crohn’s disease or sepsis which are characterized by a chronic inflammation and a high risk to develop atherosclerosis and cardiovascular diseases. One of the common features in those pathologies is the presence of low HDL levels which is associated in some cases with dysfunctional HDL, both could be markers of disease severity. Our studies on rare diseases exhibiting lipid depots in lipid-laden cells under the form of Touton cells or foamy histiocytes brought to light the major role of HDL in those pathologies in the absence of any hyperlipidemia. Indeed, we reported that the reduction of both levels and cholesterol efflux capacity of HDL particles in necrobiotic xanthogranuloma with monoclonal gammopathy (NXG) promotes macrophage lipid accumulation and could contribute to cholesterol depots into giant Touton cells observed in skin lesions characterizing NXG patients (Szalat R., 2014). Moreover, this low HDL phenotype was correlated to the unique inflammatory signature detected in NXG patients as well as to the proinflammatory phenotype of blood monocytes and to their potential ability to be recruited into skin lesions.
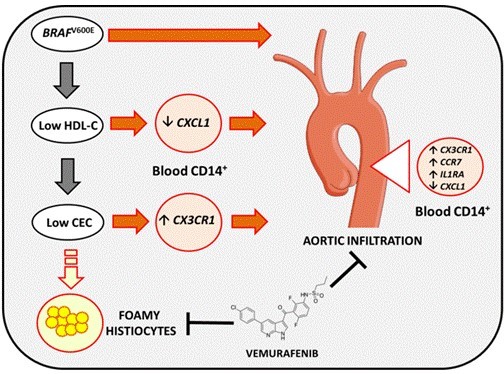
Proposed mechanisms for the lipid-laden histiocyte formation and the aortic infiltration in ECD
CEC: Cholesterol Efflux Capacity.
From Aubart-Cohen F. & al., 2018.
Our more recent works in Erdheim-Chester Disease (ECD), a rare form of non-Langerhans cell histiocytosis characterized by the infiltration of multiple tissues with foamy histiocytes, indicated that patients harboring the BRAFV600E mutation exhibit a hypoalphalipoproteinemia and a reduced capacity to promote macrophage cholesterol efflux which could contribute to foamy histiocyte formation. More importantly, cardiovascular involvement is frequent in ECD and leads to a severe prognosis and our findings uncovered a major independent role of both BRAFV600E and HDL in histiocyte infiltraton in aorta (Aubart-Cohen F., 2018).
Investigations in others forms of rare diseases associated with the presence of foamy histiocytes as well as in autoimmune diseases such as RA and SLE are currently ongoing in order to identify new physiopathological pathways and/or new lipid biomarkers associated with these diseases.
THEME 3. Lipid metabolism and cardiovascular risk – Maryse Guérin
Despite aggressive low-density lipoprotein-cholesterol (LDL-C) reduction to therapeutic goals, a majority of patients remain at high cardiovascular risk, leaving CVD among the leading causes of death around the world. Thus inhibition of the endogenous synthesis of cholesterol should be combined with alternative therapeutic strategies targeting distinct metabolic pathways. The atheroprotective role of HDL particles is related to reverse cholesterol transport (RCT) pathway, which involves the centripetal movement of free cholesterol from peripheral tissues, to the liver. In man, we demonstrated the determinant role of Cholesterol Ester Transfer Protein (CETP) activity in the modulation of biological activities of HDL particles and in particular their capacity to mediate cholesterol efflux from human macrophages (Bellanger N. 2012; Villard E., 2013; El Khoury P., 2014). We defined an optimal range of CETP activity that may represent a future clinical therapeutic goal for endogenous CETP activity in patients with metabolic disorders at high CV risk (Villard E., 2012 and 2013). We demonstrated that the overall efficacy of the RCT pathway is altered in various metabolic disorders (El Khoury P., 2016) or inflammatory states (El Khoury P., 2015) and closely associated with clinical features of atherosclerosis (Gall J., 2016). More recently, we demonstrated that serum cholesterol efflux capacity, which reflects flow of cholesterol through multiple intravascular components of the reverse cholesterol transport process, is independently associated with long term survival in myocardial infarction patients (Guerin M., 2018). These findings identified serum cholesterol efflux capacity as a useful biomarker for identification of patients at higher risk of mortality after an acute coronary event. Improving/restoring serum cholesterol efflux capacity after an acute coronary event could potentially improve patient’s prognosis. Such hypothesis is currently being tested in the large ongoing AEGIS-2 trial and supported by our findings.
Blood cholesterol is the result of input from gut absorption and endogenous synthesis, relative to clearance through hepatic and extrahepatic tissue pathways. The cholesterol metabolism is equally closely linked to bile acids metabolism as bile acids represent end-products of cholesterol that are excreted into feces which represent an additional pathway for the elimination of excess cholesterol. Physiological or pharmacological induced changes in one of these latter parameters directly impact the others suggesting that therapeutic strategies for reducing residual cardiovascular risk needs a multi-level approach.
We propose to focus on major metabolic pathways implicated in the determination of body cholesterol pool that include not only cholesterol synthesis and absorption but also its intravascular transport and fecal excretion.
Significance. Aggressive reduction of LDL-C has been the cornerstone of preventive CV care for hypercholesterolemic patients at high CV risk over recent decades. Improvement in the prevention and treatment of atherosclerosis over the past 25 years has made it possible to decrease the death rate from CVD approximately by half. However, in France, the number of deaths from CVD still amounts to almost 200,000 per year, thereby highlighting the need for identification of new and more personalized therapeutic strategies. The next decade will likely be oriented towards new approaches to reduce residual CV risk, based on the knowledge of cholesterol transport, metabolism and catabolism. A rational for combination therapies based on knowledge of the physiological pathways is necessary for a personalized medical care.
THEME 4. Postprandial state and Triglyceride Rich Lipoprotein metabolism – Jean-Marc Lacorte and Maryse Guérin
Postprandial lipid metabolism has received considerable attention since it was proposed that postprandial triglyceride-rich lipoproteins have been identified as a cardiovascular risk factor, however very few studies on postprandial lipid metabolism have been conducted. The intimate relationship between postprandial lipemia and atherosclerosis clearly suggests that the relationship between lipoprotein metabolism and atherosclerosis can no longer be considered exclusively on the basis of fasting lipid levels. An exaggerated postprandial lipemia represents a frequent metabolic abnormality of a number of lifestyle-related diseases associated with increased morbidity and mortality. Postprandial metabolism involves several organs and numerous hormones that some are dysregulated in obese or diabetic patients. In consequence identification of factors that interfere with postprandial lipoprotein metabolism is of major interest.
Transient elevation of triglyceridemia represents the first and main marker of postprandial metabolism reflecting occurrence and accumulation of triglyceride-rich lipoproteins. Thus, plasma triglyceride is the first marker used in postprandial studies. However, postprandial metabolism is equally associated with an intense intravascular remodeling of all classes of plasma lipoprotein particles. Our objective is to explore the impact of postprandial lipemia on quantitative and qualitative modification of intravascular lipoproteins of both intestinal and liver origin as well as on their anti- or pro-atherogenic properties. By high-throughput array analysis we recently identified several miRs that are differentially expressed during postprandial phase. These entirely original observations represent the first evidence that a variation of circulating miRNAs during postprandial phase is associated with a miRNAs signature that may differ according the metabolic context. We aim to determine whether postprandial miRs variations contribute into the development of CMD associated with premature atherosclerosis or might represent biomarkers of metabolic diseases.
Significance. The project will afford new insights in the comprehension of mechanisms underlying the relationship between postprandial hypertriglyceridemia and cardiovascular risk.
THEME 5. Revisiting the present concept of HDL metabolism – Anatol Kontush
Decreased plasma levels of HDL-C are established as an independent CV risk factor. This association may reflect multiple atheroprotective properties of HDL particles, including cellular cholesterol efflux from macrophages together with numerous other activities. However, large-scale clinical studies aimed to reduce CV events by raising HDL-C concentrations have failed repeatedly. Indeed, although CETP inhibitors are highly effective for increasing HDL-C (up to +150%), clinical trials of these agents brought about predominantly negative results. Furthermore, data obtained using Mendelian randomisation do not support a causal relationship between HDL-C and CV risk. Such major controversy needs to be resolved in order to allow further development of cardiovascular therapies. It is important in this regard that our recent data show that increased HDL-C levels may be paradoxically associated with increased risk of CV disease. Indeed, we found, in collaboration with other international and national teams, that a mutation of SR-BI receptor that increases HDL-C levels also paradoxically increases coronary heart disease (Zanoni, 2016).
The sources of HDL-C in the circulation are of a key importance to clarify the relationship between HDL and CV disease. A large part of HDL-C is derived, in a form of free cholesterol, from triglyceride-rich lipoproteins (TGRL), including chylomicrons and very-low density lipoproteins (VLDL), upon their lipolysis by lipoprotein lipase (LPL). This process is greatly enhanced in the postprandial phase when circulating TG levels are elevated via secretion of chylomicrons but is also active in the interprandial state. Surface fragments (free cholesterol, phospholipids, apolipoproteins) of TGRL liberated during the lipolysis add to the circulating HDL pool; ensuing esterification of free cholesterol by LCAT expands HDL particles with a formation of lipophilic core. Importantly, cholesterol efflux from peripheral cells, including macrophages, which is presently considered to represent the major anti-atherogenic activity of HDL, provides only a minor contribution to HDL-C levels, which is greatly inferior to that of TGRL lipolysis.
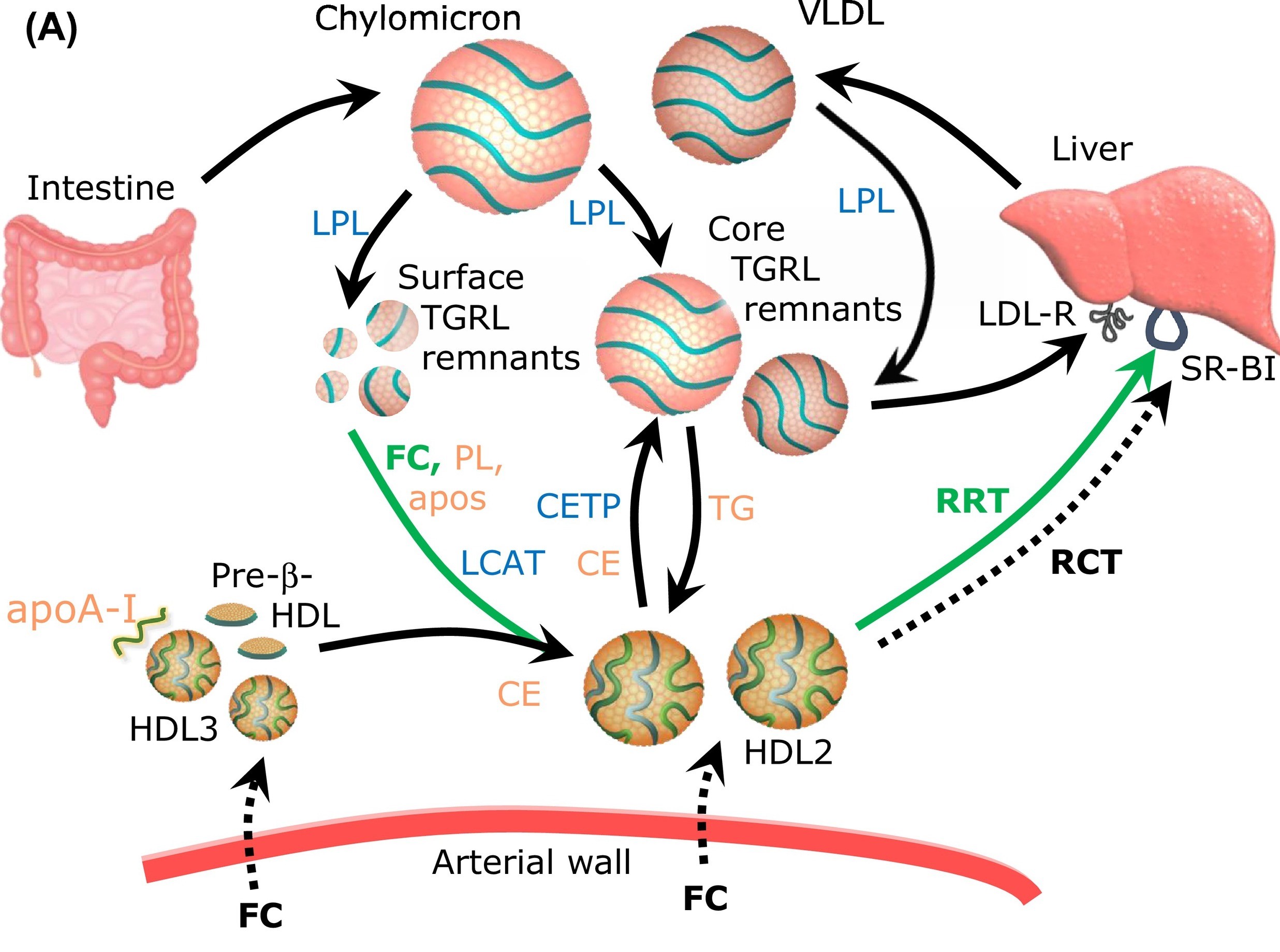
HDL prevents surface TRL remnants from accumulation in the arterial wall via acquiring phospholipid (PL) and free cholesterol (FC) during TG lipolysis.
An in vitro fluorescence-based assay to evaluate the capacity of HDL to acquire PL and FC from TRL upon LPL-mediated lipolysis.
Based on these data, we propose a working hypothesis that HDL plays a major role in the both post- and interprandial lipid metabolism, ensuring acquirement and transport of surface fragments generated during lipolysis of TGRL. Such lipid acceptor activity coupled to the transport of TGRL-derived surface lipids to the liver may represent a major function of HDL, which is presently overlooked by the mainstream research. As a corollary, HDL-C is an imperfect static measure of this dynamic pathway. In particular, low HDL-C levels associated with elevated CV risk may reflect insufficient cholesterol flux from TGRL to HDL upon lipolysis, resulting in the accumulation of cholesterol in pro-atherogenic TGRL remnants and in the development of CV disease. Lipid acceptor activity of HDL should therefore be diminished in low HDL-C subjects at elevated CV risk.
The major objective of this project is to evaluate the role of HDL in the post- and interprandial lipid metabolism as an acceptor of surface lipids liberated during lipolysis of TGRL. To achieve this goal, we primarily use a novel assay to measure lipid-accepting capacity of HDL under conditions of LPL-induced TGRL lipolysis in vitro, which was recently developed in our laboratory (European Patent EP17306042). We study mechanisms underlying the transfer of surface lipids from TGRL to HDL upon LPL-mediated lipolysis. The lipid-accepting capacity of HDL is investigated at the molecular level using modern proteomic, lipidomic and glycomic approaches to provide key information on structure-function relationships in HDL particles in both normal subjects and in patients with metabolic and CV diseases (acute MI, stable coronary disease, Type 2 diabetes, metabolic syndrome). This research is aimed at establishing clinical relevance of the assay to improve existing lipid-targeting therapies.

| Name | Position | ORCID |
|---|
- Aubart, FC, Poupel, L, Saint-Charles, F, Charlotte, F, Arsafi, Y, Frisdal, E et al.. Profound systemic alteration of the immune phenotype and an immunoglobulin switch in Erdheim-Chester disease in 78 patients from a single center. Haematologica. 2022;107 (6):1347-1357. doi: 10.3324/haematol.2021.279118. PubMed PMID:34647443 PubMed Central PMC9152974.
- Silvain, J, Kerneis, M, Zeitouni, M, Lattuca, B, Galier, S, Brugier, D et al.. Interleukin-1β and Risk of Premature Death in Patients With Myocardial Infarction. J Am Coll Cardiol. 2020;76 (15):1763-1773. doi: 10.1016/j.jacc.2020.08.026. PubMed PMID:32861811 .
- Kontush, A. HDL and Reverse Remnant-Cholesterol Transport (RRT): Relevance to Cardiovascular Disease. Trends Mol Med. 2020;26 (12):1086-1100. doi: 10.1016/j.molmed.2020.07.005. PubMed PMID:32861590 .
- Feng, M, Darabi, M, Tubeuf, E, Canicio, A, Lhomme, M, Frisdal, E et al.. Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur J Prev Cardiol. 2020;27 (15):1606-1616. doi: 10.1177/2047487319894114. PubMed PMID:31840535 .
- Amarenco, P, Kim, JS, Labreuche, J, Charles, H, Abtan, J, Béjot, Y et al.. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N Engl J Med. 2020;382 (1):9. doi: 10.1056/NEJMoa1910355. PubMed PMID:31738483 .
- Witztum, JL, Gaudet, D, Freedman, SD, Alexander, VJ, Digenio, A, Williams, KR et al.. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N Engl J Med. 2019;381 (6):531-542. doi: 10.1056/NEJMoa1715944. PubMed PMID:31390500 .
- Guerin, M, Silvain, J, Gall, J, Darabi, M, Berthet, M, Frisdal, E et al.. Association of Serum Cholesterol Efflux Capacity With Mortality in Patients With ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2018;72 (25):3259-3269. doi: 10.1016/j.jacc.2018.09.080. PubMed PMID:30573028 .
- Sturm, AC, Knowles, JW, Gidding, SS, Ahmad, ZS, Ahmed, CD, Ballantyne, CM et al.. Clinical Genetic Testing for Familial Hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2018;72 (6):662-680. doi: 10.1016/j.jacc.2018.05.044. PubMed PMID:30071997 .
- Cohen-Aubart, F, Guerin, M, Poupel, L, Cluzel, P, Saint-Charles, F, Charlotte, F et al.. Hypoalphalipoproteinemia and BRAFV600E Mutation Are Major Predictors of Aortic Infiltration in the Erdheim-Chester Disease. Arterioscler Thromb Vasc Biol. 2018;38 (8):1913-1925. doi: 10.1161/ATVBAHA.118.310803. PubMed PMID:29930009 .
- Zanoni, P, Khetarpal, SA, Larach, DB, Hancock-Cerutti, WF, Millar, JS, Cuchel, M et al.. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351 (6278):1166-71. doi: 10.1126/science.aad3517. PubMed PMID:26965621 PubMed Central PMC4889017.
- Du, XM, Kim, MJ, Hou, L, Le Goff, W, Chapman, MJ, Van Eck, M et al.. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116 (7):1133-42. doi: 10.1161/CIRCRESAHA.116.305485. PubMed PMID:25589556 .
- Frisdal, E, Le Lay, S, Hooton, H, Poupel, L, Olivier, M, Alili, R et al.. Adipocyte ATP-binding cassette G1 promotes triglyceride storage, fat mass growth, and human obesity. Diabetes. 2015;64 (3):840-55. doi: 10.2337/db14-0245. PubMed PMID:25249572 .

The HDL Handbook
1st Edition
Biological Functions and Clinical Implications
Editor: Tsugikasu Komoda
eBook ISBN: 9780123821720
Hardcover ISBN: 9780123821713
Imprint: Academic Press
Published Date: 12th August 2010
Page Count: 296
Chapter 4
HDL and Reverse Cholesterol Transport: Physiological Modulation
Giovanna Catalano and Maryse Guerin
UMRS 1166, Inserm, Sorbonne Université, Paris, France

The HDL Handbook
2nd Edition
Biological Functions and Clinical Implications
Editor: Tsugikasu Komoda
eBook ISBN: 9780124079243
Hardcover ISBN: 9780124078673
Imprint: Academic Press
Published Date: 6th November 2013
Page Count: 354
Chapter 4
Reverse Cholesterol Transport in HDL Metabolism: Modulation of Structural and Functional Features of HDL Particles
Elise F. Villard, Maryse Guerin
UMRS 1166, Inserm, Sorbonne Université, Paris, France

The HDL Handbook
3rd Edition
Biological Functions and Clinical Implications
Editor: Tsugikasu Komoda
eBook ISBN: 9780128125144
Hardcover ISBN: 9780123825137
Imprint: Academic Press
Published Date: 22nd February 2017
Page Count: 320
Chapter 5
Reverse Cholesterol Transport in HDL Metabolism: Relevance to Atherosclerosis Progression and Cardiovascular Diseases
Maryse Guerin
UMRS 1166, Inserm, Sorbonne Université, Paris, France
High-Density Lipoproteins – Structure, Métabolism, Function, and Therapeuthics
Authors: Anatol Kontush, M. John Chapman
ISBN-10 : 0470408219
ISBN-13 : 978-0470408216
Imprint: Wiley & Sons
Published Date: 20th February 2012
Page Count: 648
2. Novel assay of HDL function (EP17306042).
3. The ABCG1 gene as a marker and a target gene for treating obesity (PCT/EP2011/073140).
4. Reconstituted high density lipoprotein composition used e.g. for treating stroke, myocardial infarction, angina pectoris, pulmonary arterial hypertension and heart failure, comprises apolipoprotein and negatively charged phospholipid (EP2853259-A1).




